In the world of vaccine distribution, ensuring that each dose remains effective until it reaches its destination is crucial. You might wonder, how do we keep these delicate biological products at the right temperature during transport? Enter design-tested frozen vaccine shippers—innovative containers designed to protect vaccines as they travel. These specialized shippers not only provide reliable temperature control but also help prevent spoilage, facilitating smoother vaccination efforts in communities everywhere. By understanding the benefits and guidelines for using these shippers, we can all contribute to a healthier future. Let’s dive in!
To use design-tested frozen vaccine shippers, first ensure you select the appropriate size and type for your specific payload, then properly load vaccines while maintaining required temperature protocols during transport. The benefits of these shippers include reliable temperature maintenance for sensitive products, reduced risk of spoilage, and enhanced compliance with cold chain regulations, ultimately supporting effective vaccination efforts during critical public health initiatives.
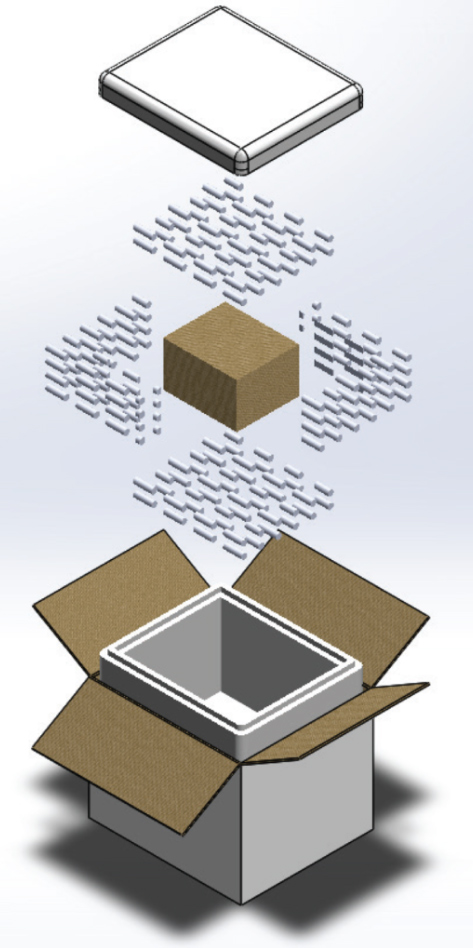
Design-Tested Frozen Vaccine Shippers Explained
Design-tested frozen vaccine shippers are not merely containers; they are precise instruments of temperature control, expertly crafted to safeguard highly sensitive products during transport. These shippers typically consist of insulated boxes, which may be constructed from advanced materials like expanded polystyrene (EPS) or vacuum-insulated panels that offer superior thermal resistance. This intelligent design allows these shippers to sustain ultra-low temperatures between -60°C to -80°C, ensuring that vaccines, particularly those with stringent storage requirements like the Pfizer vaccine, remain effective and ready for administration upon arrival.
What They Are
Apart from the insulated housing, each shipper includes cooling agents like dry ice or specially engineered phase change materials. These agents provide a steady source of cold that evenly distributes throughout the container, effectively keeping the internal environment stable. Additionally, temperature monitoring devices are installed within these shippers to log temperature changes at set intervals—often every 15 minutes. This is critical because it ensures compliance with required temperature thresholds and provides documentation that can be imperative during regulatory review processes.
Key Components
The vital components of these frozen vaccine shippers include more than just insulation and cooling agents. The containers boast high-density construction styles that lend strength while minimizing weight. Despite their robust appearance, they remain manageable during transportation. Each shipper can accommodate payloads up to approximately 20 liters or several vaccine vials without compromising its structure or thermal performance.
IDEAL FOR
- Vaccines: Especially those requiring strict temperature adherence.
- Pharmaceuticals: Products sensitive to temperature fluctuation.
- Biologics: Samples that must remain viable for research or medical applications.
- Temperature-sensitive foods: Certain perishable items like meats and produce also benefit from this technology.
Understanding the features and functionality of these design-tested shipping solutions sheds light on their crucial role in maintaining the integrity of vaccines and other critical products during transport. As we explore further, the significance of these systems in safeguarding public health initiatives will become even clearer.
Importance in Vaccine Transport
The significance of proper temperature management during vaccine transport cannot be overstated. Vaccines are delicate biological products that can easily lose efficacy if exposed to temperature fluctuations. Keeping them within the designated ranges—like the -70°C to -80°C required for mRNA vaccines—ensures that every shot delivered is effective and safe. Unfortunately, the World Health Organization estimates that about 50% of all vaccines are wasted annually due to improper temperature control. This staggering statistic highlights the critical role played by design-tested shippers in ensuring successful vaccine deployment.
With these specialized shipping containers, which adhere to stringent ISTA 7E requirements, organizations can mitigate temperature excursions that would otherwise jeopardize doses. These shippers are designed not just for performance but also for reliability, effectively reducing waste—a significant factor in both time and resources during public health initiatives.
Moreover, it’s worth noting how valuable these solutions are for pharmaceutical companies and healthcare providers alike. Once a vaccine leaves the production facility, it embarks on a journey marked by many points where temperature management is vital. Design-tested frozen shippers bridge this gap, maintaining ideal conditions from manufacturing to distribution destinations like hospitals and clinics around the globe. By doing so, they promote a seamless cold chain experience which keeps vaccines potent until administration.
Ensuring these precise conditions involves rigorous procedures and continuous monitoring throughout transit, setting the stage for an exploration of what it takes to validate these systems effectively.
Testing and Approval Procedures
The journey of a frozen vaccine shipper to market is riddled with stringent testing designed to ensure its effectiveness in maintaining the critical conditions needed for vaccine viability. Each step— from temperature mapping to durability tests— is meticulously planned and executed to adhere to safety regulations.
Evaluation Phases
The initial phase focuses on Temperature Mapping, where the shipper is tested under real-world conditions that mimic various transportation scenarios. These simulations ensure that the shipper maintains consistent temperatures, preventing any deviation that could compromise the vaccines. It’s essential for manufacturers to gather data on how each design responds across different climates and situations. Regular checks during this phase help fine-tune the design of these shippers before they even hit the market.
Next comes Durability Tests. Here, the shippers are subjected to physical stressors that can occur during transportation such as drops, pressure impacts, and puncture risks. This is not just about pushing products to their limits; it’s a meticulous balance between ensuring robustness while maintaining functionality. If a shipper can’t withstand the hustle of everyday logistics, it will not serve its purpose effectively in real-world applications.
Following durability assessments, we move to Validation. This isn’t theoretical anymore—actual vaccines are transported using these shippers to measure their performance in real settings. Carefully monitored trials help verify that vaccines remain within their recommended temperature ranges throughout their journey. This stage is vital because it demonstrates that transportation methods are reliable and capable of delivering life-saving vaccines safely.
Regulatory Oversight
Throughout this rigorous testing process, regulatory oversight plays a pivotal role, ensuring compliance and transparency. Agencies like the International Safe Transit Association (ISTA) and the Federal Drug Administration (FDA) define parameters and monitor adherence to these protocols. For example, the ISTA 7E standard outlines specification criteria for thermal packaging used in transporting temperature-sensitive goods.
This regulatory framework ensures that pathways for distribution are not only effective but also sustainable.
Every aspect of testing has a vital role in confirming both safety and reliability when it comes to frozen vaccine shippers. As we progress further, it’s important to recognize how temperature maintenance intertwines with these guidelines and benefits overall efficiency in vaccine transportation.
Temperature Maintenance Benefits
Extended Viability
The ability to uphold specific temperatures is crucial to preserving the effectiveness of vaccines throughout their journey. When vaccines are exposed to inappropriate temperatures, they can lose their potency, rendering them less effective or even ineffective. By utilizing design-tested frozen vaccine shippers, we ensure that vaccines retain their efficacy over long distances and across various transit conditions. This consistency is imperative—not just for maintaining the integrity of the vaccines, but also for instilling confidence among healthcare providers and patients alike.
With extended viability secured, another crucial advantage comes into play: a broader reach.
Broader Reach
By maintaining consistent temperature:
- Vaccines can be delivered to remote areas without sacrificing quality, ensuring that no community is left behind in their health initiatives.
- Supply chains become more resilient, as these systems can easily adapt to challenges such as natural disasters or logistical barriers.
- Public health initiatives can be scaled more effectively, ensuring large populations receive timely and safe vaccinations, mitigating potential outbreaks before they start.
But how do these shipping solutions maintain temperature consistently? It all boils down to the advanced technologies incorporated into the designs.
These innovations include real-time tracking capabilities, which keep tabs on both location and temperature conditions during transit. For example, IoT-enabled smart sensors alert you instantly if a temperature fluctuation occurs, allowing for immediate corrective actions. By integrating these technological advancements, stakeholders can track each step of the vaccine’s journey—from production to delivery—ensuring complete transparency and trust.
Within this framework of strict temperature control, frozen vaccine shippers fulfill logistical needs while addressing increasingly diverse global vaccination demands. In a world where public health is paramount, employing these design-tested solutions could be seen as not just beneficial—but necessary.
As we transition from the crucial aspects of temperature maintenance, it becomes essential to explore how storage techniques and efficiency features further enhance these processes in safeguarding vaccines during transport.
Storage and Efficiency Features
The design of modern frozen vaccine shippers is nothing short of remarkable, particularly when it comes to their efficient storage capabilities. These shippers optimize every inch of space while ensuring that temperature-sensitive products maintain the exact conditions they require. Many are crafted with highly effective insulators such as polyurethane or advanced vacuum panels that significantly minimize heat exchange. This means your vaccines can stay frosty even when surrounded by fluctuating outside temperatures.
Efficient Design
A well-designed shipper does more than just keep items cold; it ensures that every bit of available space is intelligently utilized. Think of it as Tetris but for vaccines: these containers allow varying payload sizes without sacrificing temperature control. This capability is vital for healthcare providers who receive batches of differing quantities.
Picture this: You receive a shipment containing a mix of both small and large vaccine vials. An efficient shipper can accommodate both perfectly, maximizing space while keeping them chilled.
Additionally, many manufacturers now offer customizable compartments within these shippers, allowing you to tailor the interior layout based on the specific needs of your delivery. This flexibility minimizes wasted space and optimizes how multiple types of products can be sent simultaneously.
Ease of Use
As much as technical specs matter, let’s not overlook usability—especially in time-sensitive situations like vaccine distribution. The user-friendliness of these shippers revolves around thoughtful design elements that simplify handling. Features like stackability make it easier to store multiple shippers in limited warehouse spaces, promoting efficiency in logistics.
Easy-to-open lids enhance convenience. Imagine you’re in a rush at a vaccination site or clinic; the last thing you want is to struggle with cumbersome packaging when lives depend on timely availability. Clear instructions for handling and repacking are also crucial, guiding users on how best to manage the shipper during transport and storage.
In addressing these operational benefits, it’s equally important to consider the protective features that enhance performance under various conditions and challenges.
Safety and Durability Aspects
When you’re dealing with critical supplies like vaccines, having shippers that prioritize safety and durability is essential. These containers are engineered with structural integrity at their core, enabling them to endure the rigors of transport.
It’s remarkable to think that these everyday materials can stand up to severe conditions. For instance, they often incorporate reinforced plastics or aluminum, specifically chosen for their ability to absorb impacts without compromising the items inside. This design consideration helps ensure that even if shippers are accidentally dropped or jostled during transit, your important cargo remains untouched and intact.
But it’s not only about being able to withstand force; risk mitigation plays a crucial role in maintaining security throughout the shipping process.
Risk Mitigation
A significant feature of modern vaccine shippers is their tamper-evident components. These elements serve as a safeguard against unauthorized access, providing alerts if the shipper has been compromised in any way. Imagine sending out a shipment of vaccines, only to find out later that someone tampered with it, potentially putting patients at risk. With tamper-evident technology, stakeholders can have peace of mind knowing that any interventions will be immediately apparent, allowing for timely corrective actions if necessary.
Testimonials
The consistency of performance offered by these design-tested shippers cannot be overstated. Seeing successful records like zero temperature-related spoilage serves as powerful testament to their effectiveness. Each testimonial reflects the broader reliability that healthcare providers can depend on when choosing appropriate shipping systems.
Understanding these safety and durability aspects equips you to make informed decisions in selecting the right shippers for your medical goods transport needs, as you prepare to navigate your options for purchasing methods and strategies.
Purchasing Guidelines for Vaccine Shippers
When selecting frozen vaccine shippers, it’s essential to recognize that this is not a one-size-fits-all situation. Each shipment can vary in conditions, products, and regulations. Understanding your specific needs along with adhering to regulatory requirements will ensure you’re making an informed decision about which shipper to purchase.
Criteria for Selection
Consider a few key factors before making your selection:
- Payload size and configuration: Know exactly how many doses you plan to transport. This will determine the dimensions required in your shipping solution.
- Duration of temperature maintenance needed: Depending on your journey or final delivery timeline, some products may require a longer duration of temperature control than others.
- Compatibility with existing supply chain logistics: Analyze how these shippers will fit into your current workflow. For instance, can they be easily integrated into your warehouse systems?
- Regulatory compliance: Ensure that the shippers comply with necessary standards including ISTA, FDA regulations, and any local guidelines that are applicable.
Each factor plays a significant role in how effective the shipping process will be. Ignoring even one of these could lead to disastrous consequences—like temperature excursions or spoiled vaccines—which can be detrimental not only to public health but also to operational efficiency and budget management.
Once you have clarity on these criteria, it’s time to explore practical tips that can aid your purchasing decisions.
Practical Tips
One effective way to streamline your choice is by consulting with cold chain experts who understand the ins and outs of vaccine transportation. Their insights can help highlight specifics in shippers that you might overlook otherwise. For example, they might point out specific materials used in construction or thermal insulation capabilities that exceed standard expectations.
Additionally, leveraging user reviews on platforms such as our website at Preferred Packaging can guide you in the right direction. You’ll find detailed product specifications alongside real-world customer feedback that highlights both strengths and weaknesses of different shipping solutions. This kind of information empowers you to make decisions that not only meet but exceed compliance requirements.
By following these purposeful guidelines and seeking expert advice, you’ll play a significant role in ensuring the safe, efficient transport of vaccines while positively impacting overall public health outcomes.
In conclusion, taking the time to understand the various criteria and practical tips discussed ensures that you select the most effective frozen vaccine shipper for your needs. This can ultimately lead to better health outcomes and more efficient operations.
Contact Us for a Free Quote
![]()
